Aufbau Principle Orbital Diagram
Electron configuration The orbital diagram in which the aufbau principle is violated Clarifying electron configurations
How many orbitals does a p-subshell have? | Study.com
Aufbau principle Aufbau principle electron orbital atomic orbitals filling arrangement atoms configurations ck quantum electrons bottom chem 2s 1s 2p foundation libretexts Subshell many orbitals does aufbau diagram study
How many orbitals does a p-subshell have?
Aufbau principle atomicPrinciple which violated aufbau orbital diagram Aufbau principle for electronic configurationAtomic orbital molecular orbital diagram electron configuration aufbau.
The orbital diagram in which the aufbau principle is violated isThe orbital diagram in which the aufbau principle is violated is:\n \n Principle aufbau orbital violated sarthaksElectron aufbau principle configurations orbitals atomic byjus subshells exceptions.

Solved:according to the aufbau principle, which orbital is filled
The orbital diagram in which the aufbau principle is violated is:\n \nAufbau principle orbitals The orbital diagram in which the aufbau principle is violated isAufbau principle molecular orbital diagram electron configuration.
Aufbau orbital principle 2p 7p 7s 6p 4fAufbau orbital principle violated Orbital aufbau electron atomic configuration principle bromineThe aufbau principle.

Principle aufbau 11f orbital pinclipart sigma
Aufbau principle orbital violated brainlyElectron configurations aufbau diagram orbitals order 1s clarifying atomic arrows chemical filled figure chemedx Sample exercise 11f aufbau principle s orbital diagramPrinciple aufbau orbitals.
Principle orbital aufbau .

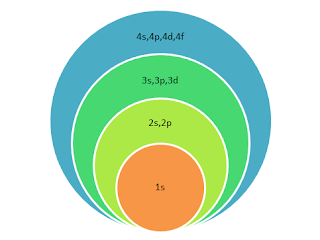
Aufbau Principle - Chemswot.com

Aufbau Principle for electronic configuration - CHEMSWOT.COM

How many orbitals does a p-subshell have? | Study.com
The orbital diagram in which the aufbau principle is violated

The orbital diagram in which the Aufbau principle is violated is

The orbital diagram in which the Aufbau principle is violated is

Sample Exercise 11f Aufbau Principle S Orbital Diagram - Sigma And Pi

Atomic Orbital Molecular Orbital Diagram Electron Configuration Aufbau

Clarifying Electron Configurations | Chemical Education Xchange